Karvol capsules banned: Reasons, Safety Concerns, and Alternatives
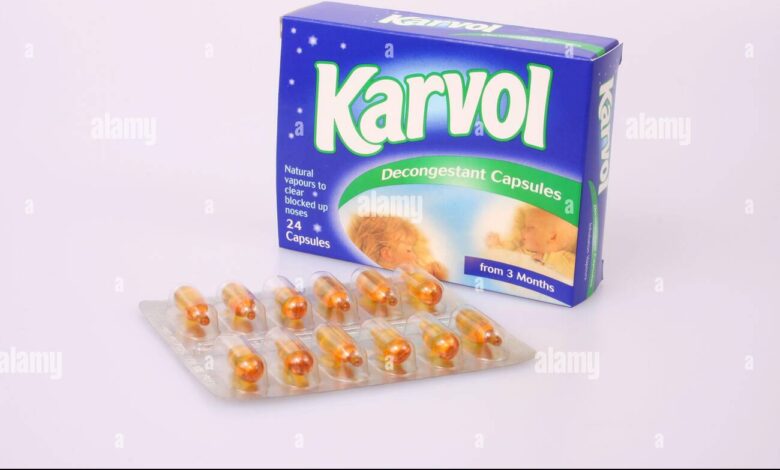
For decades, Karvol capsules were a familiar remedy in medicine cabinets across households. Known for their distinctive aroma and quick relief from nasal congestion, colds, and minor respiratory issues, these capsules were trusted by millions of families. Whether it was a blocked nose on a winter night or seasonal allergies causing discomfort, many people relied on Karvol as their go-to solution. The convenience of snipping open a small capsule and inhaling the vapor made it both easy and effective in the eyes of consumers.
However, the once-popular remedy became the subject of growing concern, leading to regulatory authorities banning its sale in many regions. The phrase “Karvol capsules banned” has since generated curiosity and concern, especially among those who grew up using the product. What exactly led to this decision? Was the ban justified, and are there safer alternatives today?
This article explores the history, composition, and eventual ban of Karvol capsules banned. We’ll also examine the health concerns raised by experts, the role of regulations, and what consumers can use instead. By the end, you’ll have a full understanding of why Karvol was pulled from shelves and what lessons it offers about over-the-counter remedies.
What Are Karvol capsules banned?
Karvol capsules banned were small, soft gelatin capsules filled with a concentrated blend of aromatic oils. Their formulation typically included ingredients such as menthol, camphor, pine oil, chlorothymol, eucalyptus oil, and terpinol. These ingredients were known for their strong decongestant and soothing properties, providing instant relief when inhaled. The method of use was simple: snip open a capsule and either inhale the vapors directly or add them to a bowl of hot water for steam inhalation.
The appeal of Karvol lay in its accessibility and natural base. Unlike strong prescription medicines, it was available over the counter and marketed as a safe option for both adults and children. Parents often turned to it when their children suffered from blocked noses, especially at night. Many users also believed in its ability to promote better sleep by clearing nasal passages.
Across countries like the UK, India, and South Africa, Karvol capsules banned became part of household traditions. Their distinctive green packaging and sharp scent made them instantly recognizable. Over the years, advertisements positioned Karvol as a gentle yet effective solution, reinforcing consumer trust.
Yet behind its popularity lay certain risks that weren’t fully appreciated until much later. While many adults used Karvol without issue, health authorities began to raise concerns about safety, particularly in vulnerable groups like children and infants. These concerns eventually paved the way for the product’s ban in several markets.
Why Were Karvol Capsules Banned?
The ban on Karvol capsules banned did not happen overnight. It was the result of growing scrutiny from medical experts, regulatory authorities, and consumer safety watchdogs. At the center of the issue were concerns about the toxic potential of certain ingredients, particularly camphor and eucalyptus oil, when misused or ingested accidentally.
One of the major factors was the risk to children. While Karvol was often marketed as safe for use in pediatric care, reports emerged of accidental ingestion by young children. Even small amounts of camphor can be toxic, causing symptoms such as nausea, vomiting, seizures, and in severe cases, respiratory failure. Infants and toddlers were especially vulnerable, as they were more likely to mistake the capsules for something edible.
Another issue was the lack of strict dosage control. Since the capsules were simply cut open and inhaled, users often determined the intensity themselves. This raised the possibility of overexposure, particularly in cases where capsules were applied directly to pillows or bedding. Continuous inhalation of concentrated vapors could irritate the respiratory system and pose risks to those with asthma or sensitive airways.
Regulatory bodies such as the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) eventually intervened, citing safety concerns. The decision to withdraw Karvol from shelves was not universally implemented at the same time across all countries, but markets like the UK saw it phased out as part of consumer protection measures. While some users protested the move, health experts argued that the risks outweighed the benefits, especially when safer alternatives were readily available.
Health Concerns Linked to Karvol capsules banned

The health risks associated with Karvol capsules banned were the main driving force behind the ban. Camphor, one of the key ingredients, is well-documented for its toxic effects if ingested even in small amounts. Medical literature records cases of camphor poisoning, particularly among children, leading to seizures and hospitalization. The brightly colored capsules only added to the risk, as young children could easily confuse them with sweets.
Another ingredient of concern was eucalyptus oil, which, while widely used in aromatherapy, can also be toxic in concentrated doses. Inhaling it excessively or applying it too close to infants’ noses could cause respiratory distress. Pine oil and menthol, though generally safe in controlled amounts, also carried risks when overused. Skin irritation, eye discomfort, and mucous membrane sensitivity were among the reported side effects.
Doctors highlighted that while adults might tolerate these compounds reasonably well, infants and children were at a much higher risk. Pediatricians particularly warned against using Karvol for children under two years old. Yet, in many households, Karvol remained a go-to remedy for young children until the ban drew attention to these dangers.
Beyond toxicity, the lack of consistent medical guidance also contributed to the problem. Because Karvol was marketed as an over-the-counter remedy, many users never sought professional advice before using it. This contributed to misuse, overuse, and in some cases, severe health consequences.
The ban, therefore, was not only about preventing individual cases of harm but also about setting a precedent that consumer health and safety must take priority, even when a product has been popular for decades.
Alternatives to Karvol capsules banned for Congestion Relief
For those who once swore by Karvol, its absence from pharmacy shelves may have left a gap. Fortunately, there are safe and effective alternatives that provide relief from nasal congestion and colds without the associated risks.
One of the simplest and safest methods is steam inhalation with plain water. Adding a few drops of doctor-recommended essential oils like peppermint or diluted eucalyptus oil can enhance the effect, though these must be used cautiously, especially with children. Saline nasal sprays and drops are another safe option, suitable for adults and children alike. They help moisturize nasal passages and clear mucus without the risk of toxicity.
For children, pediatricians often recommend saline drops, humidifiers, and natural remedies like warm honey water (for those over one year old). These remedies provide comfort without exposing children to potent essential oils. Over-the-counter decongestant tablets or nasal sprays may also be recommended for adults, though these should be used under guidance to avoid dependency or rebound effects.
Natural remedies also hold a place. Ginger tea, turmeric milk, and honey are well-regarded in traditional medicine for soothing cold symptoms. While they may not clear congestion as quickly as Karvol, they offer relief without significant risks.
The key takeaway for consumers is that safer alternatives exist, and choosing remedies backed by medical guidance ensures both effectiveness and peace of mind. The end of Karvol does not mean the end of quick relief—it simply means making better-informed choices.
Broader Lessons from the Karvol Ban
The Karvol ban offers broader insights into the role of regulations, consumer safety, and the importance of informed health choices. It shows that even widely used and trusted remedies can pose risks that only come to light after years of use. Regulatory authorities are tasked with balancing consumer demand against public safety, and in this case, protecting vulnerable groups like children took precedence.
The ban also highlights the need for consumers to remain cautious about over-the-counter remedies. Just because a product is available without prescription does not mean it is free of risks. Reading labels carefully, understanding ingredients, and consulting doctors are essential steps in responsible healthcare.
For manufacturers, the Karvol case underscores the importance of transparent labeling, childproof packaging, and clear usage instructions. Many of the risks could have been mitigated with stricter safety measures. Going forward, pharmaceutical companies must prioritize safety alongside effectiveness.
In the bigger picture, the story of Karvol reflects a growing awareness in society: natural or herbal remedies are not always harmless. Critical evaluation, supported by medical science, remains crucial in ensuring the well-being of consumers.
Conclusion
The banning of Karvol capsules marked the end of an era for a product that once held a trusted place in households around the world. While beloved for its effectiveness in relieving nasal congestion, the risks associated with its ingredients, particularly for children, made the decision necessary from a public health perspective.
Today, safer alternatives exist that offer the same relief without the dangers. Steam inhalation, saline sprays, and doctor-approved remedies provide effective options for both adults and children. The Karvol story serves as a reminder that safety should always outweigh nostalgia when it comes to health. By understanding the reasons behind such bans, consumers can make more informed and responsible choices.
FAQs About Karvol Capsules Ban
Why were Karvol capsules banned?
They were banned due to safety concerns, particularly risks of camphor poisoning and accidental ingestion by children.
Are Karvol capsules still available anywhere?
In many countries, they have been withdrawn from sale. Availability may vary regionally, but they are no longer widely marketed.
What were the main ingredients in Karvol capsules?
They typically contained menthol, camphor, pine oil, eucalyptus oil, chlorothymol, and terpinol.
Are there any health risks if I still have old Karvol capsules at home?
Yes. Expired capsules can lose effectiveness, and accidental ingestion remains a risk, especially for children. It is best to dispose of them safely.
What can I use instead of Karvol capsules for congestion relief?
Safe alternatives include saline nasal sprays, steam inhalation, humidifiers, and doctor-recommended medications.
Was the ban global or only in certain countries?
The ban was not global. However, countries like the UK withdrew Karvol capsules due to regulatory concerns.
Are there similar products still available on the market?
Yes, some vapor rubs and inhalants are available, but they are often reformulated with stricter safety standards. Always check labels and consult healthcare professionals.
You May Also Read: John Peel paedo




